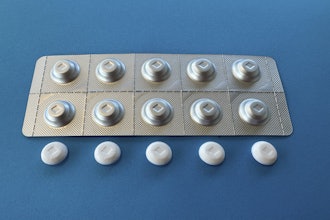
Brains Bioceutical has claimed a "significant breakthrough" in what it's calling the world’s first solid Tetrahydrocannabinol (THC) as an Active Pharmaceutical Ingredient (API).
"As a scientist deeply involved in cannabinoid research, I am thrilled by Brains Bio announcement of the solid D9 THC API. This innovation not only represents a significant leap forward in the pharmaceutical application of cannabinoids but also underscores the critical need for high-quality, reliable APIs in advancing clinical research. The solid form of THC as an API paves the way for more precise dosing, improved stability, and broader application in drug development, addressing some of the key challenges we face in cannabinoid therapeutics today. It's a milestone that promises to accelerate our quest for effective cannabinoid-based treatments, offering new hope for patients worldwide. I commend Brains Bioceutical Corp for their commitment to excellence and innovation in this rapidly evolving field." Dr. Ichiro Takumi, M.D., Ph.D, Chairman of the board of directors, JCAC (Japan Clinical Association of Cannabinoids), Professor of Neurosurgery, St Marianna University School of Medicine
To highlight the significance of this milestone, Brains Bio has shipped solid D9 THC samples to researchers and pharmaceutical companies in Asia and South America. The company said the product could help pharmaceutical research and treatment options in various therapeutic areas, including pain management, neurological disorders, and mental health. The introduction of the solid D9 THC underscores Brains Bio role in pioneering new therapies and enhancing patient care through scientific discovery.